COVID-19 Vaccines: A Rundown of What is Going Down
The flurry of activity in recent weeks on the COVID-19 vaccine front has led to a mix of excitement, relief and some hesitancy as vaccine approvals and doses are being churned out at unprecedented speed.
So far, COVID-19 vaccines developed by German biotech company BioNTech (Biopharmaceutical New Technologies) in partnership with American pharma giant Pfizer (BNT162), and one developed by Massachusetts-based Moderna (mRNA-1273) have made the cut, having received emergency use authorizations (EUA) from the US Food and Drug Administration (FDA).
Health Canada has given approval to BioNTech-Pfizer’s vaccine, with one for Moderna’s expected to soon follow.
Among other vaccine front-runners, AstraZeneca-The University of Oxford and Johnson & Johnson are also looking to win regulatory approvals for their vaccine candidates.
As we head towards getting COVID vaccines, people are on the edge of their seats wondering how they work and if they will be the magic key to ending the pandemic. Leave it to the experts say the experts, while conspiracy theorists are beating drums about microchip-laced vaccines and something about people and sheep.
Overall, the arrival of pandemic-ending vaccines onto our shores is being met with resounding sighs of relief. Encouragingly, in a recent US survey, 84 percent of people say they will get the vaccine, with about half in the group opting to wait it out a bit. This is a win for helping end the pandemic and the science that got us there.
In the same survey, less than 15 percent of respondents said they will never get the vaccine while 39 percent think states should make vaccination mandatory.

But a vaccine is not a magic wand that will end the pandemic overnight. 2021 will only be the beginning of what still looks like a decent-sized road to the end of the pandemic.
While vaccines cam protect you from getting seriously sick from a virus, they do not prevent you from getting it.
While it remains unknown whether any of the COVID-19 vaccines in development can reduce transmission, what is known is that their primary function is to reduce severe illness and death (which they’ve been shown to do effectively in clinical trials, with efficacies around 94 to 95 percent). As such, the most vulnerable and high-risk populations — namely the elderly and staff at long-term care homes, as well as frontline healthcare workers — are being prioritized for vaccination.
This is critical as current COVID-19 mortality rates have stood at an average of nearly 1,000 deaths per day in the US since the first COVID-19 case was identified in the country. That number is now 3,000 per day, making the novel coronavirus one of the leading causes of death in the country.
For people who like to compare the number of COVID-19 deaths to those resulting from the flu or car accidents, it is important to note that this is an excess number of deaths per day. Excess = extra, additional.
And what they should more importantly understand is that pain, suffering and avoidable deaths should be prevented under any and all circumstances.
To put it into perspective, the flu kills between 12,000 and 61,000 people each year in the US, while COVID-19 has killed over 300,000 in less than a year. That’s about five times higher than the flu in its worst seasons.
If COVID-19 has a mortality rate of even 1 percent (although estimates are closer to 3 percent), that’s over 3 million deaths in the US (in a population of just over 300 million, and assuming everyone gets infected. A large number would if pandemic measures were not in place). A lot of these deaths can be simply and easily prevented with masks and lockdowns. And without the current measures we have, the deaths would be a lot higher.
It is also important to be aware that while vaccines protect you from getting seriously sick from a virus, they do not prevent you from getting it.
Therefore, even after vaccination, you can still get the SARS-CoV-2 coronavirus that causes COVID-19 and pass it to others. This has obvious implications for transmission, which may or may not be modified by vaccines. Vaccine data on transmission is still early so it is premature to say anything about their impact on transmission.
The work we have all collectively done so far — which has included an arsenal of masks, physical distancing, frequent handwashing, lockdowns, as well as evaluating failures in long-term care, marginalized communities and schools — must continue.
This is not unique to a COVID-19 vaccine, it’s how all vaccines work, which is why mass vaccination campaigns are employed to help eradicate communicable diseases within a population. Simply put, vaccines work best when everybody gets them.
Therefore, until enough people are vaccinated, the work we have all collectively done so far — which has included an arsenal of masks, physical distancing, frequent handwashing, lockdowns, as well as evaluating failures in long-term care, marginalized communities and schools — must continue. This is particularly important as case numbers continue to soar, hitting record highs in many places in North America.
How Vaccines Work
Your existing, non-SARS-CoV-2-exposed immune system will not protect you against COVID-19.
Vaccines deliver a sampling of a virus for the body to use to create a tailored defense system (immunity) against it. The virus is either weakened, ‘killed’ or the vaccine only contains a part of the virus in DNA, mRNA or protein form.
This defense system consists of Y-shaped proteins called antibodies and immune cells called lymphocytes (a type of white blood cell) that include fighter T cells and memory B cells — the latter create a ‘memory’ of the virus so they can quickly produce antibodies against it in any future attack from it.
Antibodies are a part of the humoral arm of the adaptive immune system, which consists of molecules circulating in fluids such as the blood. Immune cells are part of cell-mediated adaptive immunity.
There are an estimated 10 billion different types of antibodies in the body. Each recognizes a different antigen (a small protein molecule), including those from pathogens like viruses and bacteria.
Similarly, there are millions of different types of T cells, each specific to a particular antigen. T cells generally persist in the body longer than antibodies. They may therefore be a better system and measure of disease-specific immunity.
Your existing immune system is comprised of specific antibodies and cells against pathogens it has encountered. So your existing, non-SARS-CoV-2-exposed immune system will not protect you against COVID-19, contrary to what some people believe. You need to be exposed to the virus to build immunity against it, either through natural infection or a vaccine.
Vaccination Primarily Protects You
Someone who has been vaccinated should theoretically have lower viral loads (amount of measurable virus in a given material such as a blood sample) if they end up contracting the virus in question. This is because their vaccine-generated defense system would destroy the virus, preventing it from replicating and making copies of itself.
However, the primary action of vaccines is to prevent infection, not transmission.
Nevertheless, data from Moderna’s candidate vaccine trial shows that the company’s vaccine could potentially prevent asymptomatic infections, which would have positive implications for transmission.
SARS-CoV-2 is a novel virus that we’re still learning about it, including how much of a viral dose is needed to cause infection and/or symptoms. A recent study has shown that higher viral loads are associated with worse disease severity and an increased risk of mortality.
We also don’t know how long immunity conferred by a vaccine will last, nor immunity after a natural infection. While there have been some reports that antibodies against SARS-CoV-2 may only last for six months in the body, new research shows that immunity from T cells and B cells is much longer and could last for years.
Regardless, and until we have more data, masks will have to stay put until a significant number of people are vaccinated, enough to achieve herd immunity.
Herd Immunity
The concept of herd immunity is that transmission of a virus decreases when a significant proportion of a population has immunity against a pathogen. This is because there are less bodies that a virus can take up residence in and use to propagate itself. If 80 percent of a population has immunity against a virus, the idea is that the remaining population will be indirectly protected because the virus will be met with more immune bodies than non-immune ones, lowering infection probability.
While estimates generally range somewhere near 70 to 80 percent of a population needing to be vaccinated for herd immunity (widespread, community-level immunity), these percentages vary across diseases.
For example, approximately 95 percent of a population needs to be vaccinated for herd immunity against measles (considering that the remaining five percent will be protected by those that are vaccinated) while for polio, that percentage is about 80 percent.
The term herd immunity has been used and abused in rather awful fashion during the pandemic, with some waywards thinking that letting the virus run rampant and infecting everyone is how to go about things. Inflicting human suffering and death in this way is a despicable thought.
Mutations
It is normal for viruses to mutate as they adapt to selection pressures in their environment. The influenza A and B viruses that cause the seasonal flu are notorious for mutating every year, which is why the flu vaccine is changed yearly to target the changing strains. Coronaviruses have a much lower mutation rate (by as much as 50 percent) than the influenza viruses and thus experience far less mutations.
Recently a new variant of the SARS-CoV-2 coronavirus was identified in the UK, and so far it has only been found in one other country (Denmark).
The new variant has two mutations in the spike protein — the projections on the surface of the virus that are essential for its binding to host cells for entry; most of the vaccines being developed against SARS-CoV-2 target this protein. The spike proteins give the virus a crown-like appearance, and the word ‘corona’ is the Latin word for crown. The spike protein binds to a receptor on human cells called ACE2 (angiotensin-converting enzyme 2).
The new variant is called N501Y, referring to one of the mutations where the amino acid asparagine (N) has been substituted with, or changed to tyrosine (Y) at the 501 position in the protein sequence of the spike protein. It is thought that this mutation may allow for tighter binding of the spike protein to the ACE2 receptor. The other mutation in the same variant is a deletion of amino acids at positions 69 and 70 and may be associated with evasion of the immune response (observed in immunocompromised patients).
It is being reported that the new N501Y variant may be up to 50 percent more transmissible, but not necessarily able to cause more severe disease.
In the summer, two different variants of the virus were found in Spain, which ended up spreading across Europe.
So far the emergence of a new strain of the virus is unlikely to have major implications from a vaccine perspective. This is because the vaccine is designed to build immunity against more larger parts of the spike protein. As such, a low number of mutations that do not significantly alter the structure or function of the protein may not affect the ability of vaccine-generated antibodies to recognize it.
However, if the mutations affect the structure of the spike protein, that could mask epitopes (specific spots on the protein) that the vaccines are designed to target. It is still too early to say this with any certainty as researchers are closely following and studying the new variant and other circulating strains.
Separating Fact from Fiction
Evidence-based medicine and science are premised on fact checking evidence through internal standards (i.e. ensuring data has statistical significance, for example) and external peer-review processes.
The Oxford Dictionary’s definition of fact is “a thing that is known to be true, especially when it can be proved.”
The last time I checked, an evidence-less conspiracy theory does not have an iota of fact that can be checked, much less proven.
Below are some common points, questions, concerns and conspiracies that are coming up as we have reached the critical milestone of having a COVID-19 vaccine at our doorsteps.
So Fast?
The latest tools to study diseases and develop therapies — including mRNA vaccines — were available and in place long before any coronavirus pandemic came knocking.
It typically takes ten years to develop a new vaccine or drug — from writing research proposals for funding, stirring things up in a test tube in a lab, testing it in animal models (preclinical testing), trials in humans (clinical testing), through to obtaining regulatory approvals and delivery to a store or clinic near you.
For COVID-19, this timeline was cut to just ten months. This was made possible by bypassing a lot of the bureaucracy involved in drug development (applications sitting on desks and collecting dust until the 20th email follow-up), allowing for quick development and approvals. The pandemic necessitated the speed.
We Had the Latest Science and Technologies
Significant advances and improvements in biomedical research technologies and computational data analysis tools have been made in the past decade.
For example, genetic sequencing has improved by leaps and bounds since the days of the Human Genome Project in the early 1990s, which aimed to sequence and decode the entire human genome. The project took almost 13 years to complete from start to finish. Now with new next-generation sequencing technologies, this can be achieved in a matter of hours and days.
This is what enabled the sequencing of SARS-CoV-2’s genome within just a day or so by Chinese researchers, who made its sequence publicly available in early January, kickstarting COVID-19 vaccine development around the world.
In addition, high-throughput molecular technologies allow researchers to quickly identify biological targets and design compounds against them.
Companies like BioNTech and Moderna have been working on mRNA technologies for over ten years. With the technology on hand, Moderna was able to develop a prototype COVID-19 vaccine in just two days.
BioNTech was founded in Germany 12 years ago by Turkish husband and wife duo Uğur Şahin, MD, PhD and Özlem Türeci, MD. The company focuses on developing mRNA-based drugs and vaccines for cancers, and was able to seamlessly add a COVID-19 vaccine pipeline to its portfolio when the need arose.
Moderna has been in the mRNA vaccine arena for nearly a decade. It was founded in 2010 by Canadian stem cell biologist Derrick Rossi, PhD, Associate Professor in the Stem Cell and Regenerative Biology Department at Harvard Medical School in Massachusetts, US. The company has worked to develop an mRNA platform that can be leveraged to make vaccines for various diseases and conditions.
Funding
With the latest mRNA technology platforms on hand to plug in a code for a vaccine against SARS-CoV-2, BioNTech/Pfizer and Moderna received ample funding from governments and private investments for their COVID-19 vaccine programs.
As opposed to applying for grants and pitching to investors, which is a time-consuming process that can take upwards of a year or two, funding was readily provided by country governments, health agencies and private investors around the world, in light of the pandemic emergency. The situation was, ‘we’re in a pandemic, we need vaccines now, here is the money, now go do it.’
The US’s Operation Warp Speed program alone provided $1 billion in funding for COVID-19 vaccine development.
Efficient Participant Recruitment for Clinical Trials
Being in a pandemic enabled large numbers of participants for COVID-19 vaccine clinical trials to be quickly recruited into clinical trials as there was a high chance that there would be exposure to the coronavirus given its active circulation in communities worldwide. As such, tens of thousands of people were enrolled in Phase III clinical trials very quickly. This allowed for clinical endpoints to be met within just months in the trials, as statistically significant data could be yielded quickly given the large numbers of people that the studies had.
Cutting the Bureaucracy and Red Tape
There is typically a lot of bureaucracy involved in drug and vaccine development. That’s why it can take up to ten years from the inception of a drug through to getting it to market.
Applying for funding through grants can take well over a year or more. After the drug or vaccine has been developed and has gone through extensive pre-clinical in vitro and in vivo testing, i.e. testing in a dish and in animals, respectively, it is ready to be tested in humans in clinical trials. Getting approvals for clinical trials involves extensive applications and paperwork that can sit around on the desks of regulatory agencies once submitted. There are a lot of administrative details involved that can make it a lengthy process.
After a clinical study is approved, the trial can begin. Depending on the trial, data can be submitted to regulatory agencies on a rolling basis for review, but this is not usually the case. Data is typically submitted once a trial is fully complete and has met all of its primary and secondary endpoints. And once data is submitted to regulatory boards, agencies can take their sweet time reviewing the data before making decisions and finally granting any kind of an approval.
All in all, there are a lot of ‘middle people’ and red tape involved in the drug development process. Case in point, medications like Tylenol and Advil did not take ten years to develop because they were being tested for that long in humans. It was because funding application, review and approval processes took up much of that time because of all the bureaucracy involved.
For the COVID-19 vaccines, time was of the essence. Therefore, any unnecessary logistical and bureaucratic hindrances were removed to allow for a swift development path.
Rapid Regulatory Pathways and Reviews
Regulatory programs and pathways such as emergency use authorization (EUA) and fast-track approval pathways at regulatory health agencies like the US FDA are not new. The EUA program itself is designed to address public health emergencies like pandemics so that potentially life-saving treatments can be quickly authorized for emergency use.
For the COVID-19 vaccines, independent data monitoring committees reviewed clinical trial data on a rolling basis as it became available, as opposed to waiting until the trial ended. Once clinical endpoints were met and applications for authorizations and approvals were submitted to regulatory agencies, the agencies worked around the clock to meticulously review the final data and make the decisions. Again, cutting out bureaucracy, middle people and red tape allowed for things to move fast, but not rushed.
Takeaway
The main takeaway is that the latest tools to study the virus and the disease it causes, and to develop vaccines against it, were available and in place long before the COVID pandemic came knocking at our doors.
As the pandemic has shown us, faster timelines for drug and vaccine development are possible given the correct fuel and fire. The hope is that this real-world pandemic example may help spur shorter and more efficient timelines for the development of treatments for other diseases and conditions.
In the end, the COVID-19 vaccines were not rushed and steps were not skipped. They were funded, tested and reviewed rigorously, in a focused and no frills manner.
Is it Safe and Does it Work?
The rapid development of COVID-19 vaccines has some questioning their safety and efficacy. Safety and efficacy are in fact two primary endpoints — measurable outcomes that are used to determine whether treatment is beneficial — in clinical trials.
Safety endpoints assess whether a treatment intervention is safe and has a low risk of inducing any potential harm. Safety monitoring is in fact conducted throughout all phases of a clinical trial to identify, manage and minimize risks associated with the under-investigation agent.
Efficacy evaluations look at whether an agent works to produce the expected result for the intended purpose. In clinical trials for vaccines against SARS-CoV-2, the major efficacy endpoint was the prevention of illness, particularly severe illness.
The COVID-19 vaccines from BioNTech/Pfizer and Moderna had reported efficacies of over 90 percent in results from their Phase 3 clinical trials. For a vaccine to be deemed effective, the FDA requires an efficacy of at least 50 percent.
After Phase 1 safety testing and dose determination, the investigational treatment enters Phase 2.
A clinical trial program has four phases.
Phase 1 is designed to evaluate the safety of a vaccine or drug through dose escalation studies. These studies involve increasing the dose of an agent to levels that are safe and tolerable. Once the safest dose has been determined, that dose is used in the next set of clinical studies.
Phase 2 involves anywhere between a handful of participants up to a few hundred. Phase 2 trials continue to monitor safety with initial insights into efficacy.
Phase 3 is a large-scale randomized control study that is typically conducted across multiple health centres and sites, and involve thousands of people. The focus of Phase 3 trials is efficacy, with safety and side effects closely monitored throughout as well.
Phase 4 studies are typically post-marketing surveillance trials which continue to monitor safety and efficacy after an agent has been approved and made available to the public. The trials may also involve expanding testing to more populations such as children and pregnant women.
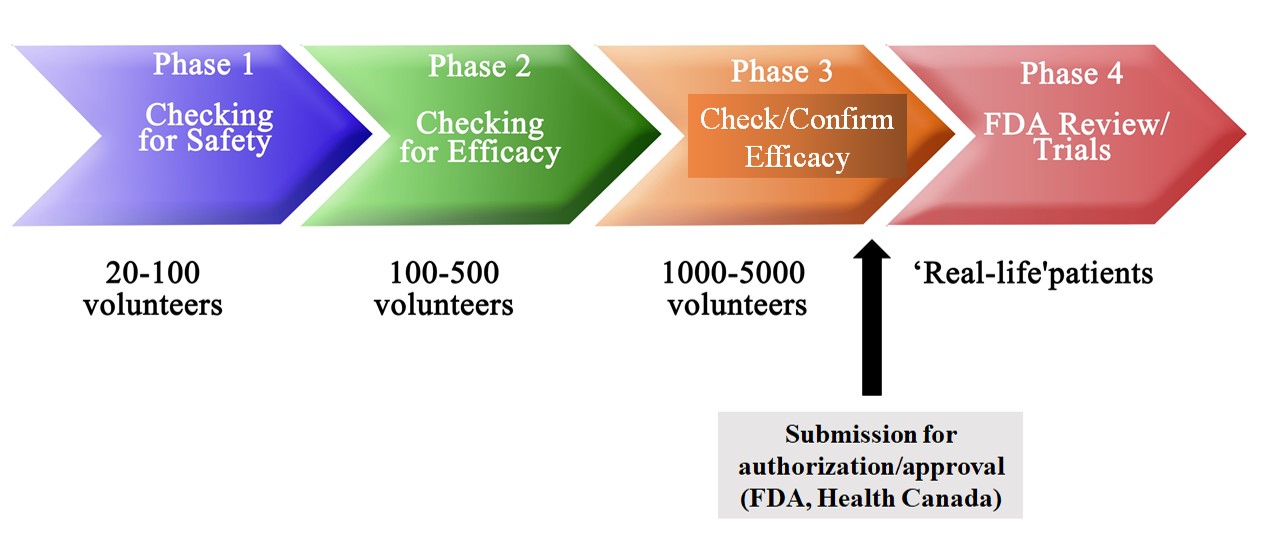
Trials are monitored by independent data monitoring committees, which are comprised of independent scientists who oversee the safety and scientific integrity of a clinical study. These committees check in at regular intervals to evaluate trial progress and collected data.
AstraZeneca in fact had to halt its trial in September after an adverse neurological side effect was reported. An independent data monitoring committee conducted an investigation into the case after which clearance was given for recommencement of the study.
Not Enough Testing
There is a concern among some that the vaccines haven’t been tested long enough, or among enough people.
9 Months to Delivery
The first COVID-19 vaccine was administered on March 16 to a volunteer as part of Moderna’s Phase 1 safety trial conducted at Kaiser Permanente Washington Health Research Institute in Seattle. The individual has been monitored for nine months since then and continues to be followed (follow-up typically continues for one or two years).
No serious adverse reactions to the vaccine have been reported in the volunteer thus far. Any major adverse side effects to a vaccine occur within hours of injection, and at most up to three months.
Large Numbers of People
When you get to a Phase 3 clinical trial, as the major vaccine contenders did, you are talking large numbers and minimum monitoring timelines of two months.
The randomized, placebo-controlled Phase 3 trials conducted by BioNTech/Pfizer and Moderna and AstraZeneca/Oxford have involved 30,000 participants at minimum, with Pfizer’s trial having 43,448 to be exact. In the trial, half of the group received the vaccine and the other half received placebo. The trial showed that the vaccine was well tolerated and had an efficacy of 95 percent in preventing COVID-19 in those without any previous infection seven days or more after the second dose.
Efficacy results came from a smaller group of 170 people who contracted COVID-19, among which only eight cases occurred in the vaccinated group and 162 in the placebo arm.
In the first interim analysis conducted for Moderna’s Phase 3 trial called the COVE study, five cases of COVID-19 were observed in the vaccine group versus 90 in the placebo group, resulting in a vaccine efficacy of 94.5 percent.
A secondary endpoint of the trial involved analysis of severe COVID-19 cases. All severe cases (11) occurred in the placebo group and none in the vaccinated group, demonstrating 100 percent efficacy of the vaccine in protecting against the development of severe COVID-19 illness.
The more controversial challenge trial format involves giving the virus to people, which could help accrue a larger experimental group, but has obvious risks associated with it.
Varied Demographics
Both BioNTech/Pfizer and Moderna’s Phase 3 trials included people of varied demographics, including older adults as well as people of different racial backgrounds and with different health conditions and co-morbidities, with results being consistent across different subgroups. BioNTech/Pfizer’s vaccine was particularly effective in older adults, which is promising as they are at greater risk of getting seriously ill from COVID-19. Historically, most drug and vaccine trials have typically been conducted among healthy, Caucasian adults between the ages of 18 and 55.
Mandatory Data Presentation
COVID-19 vaccine makers were required to present two months of post-vaccination data (after the second dose for vaccines with dual injections), and trial participants will continue to be monitored even after vaccines are administered to the general population. This would potentially cause the placebo, non-vaccinated group to get smaller as people in that arm get vaccinated.
Other Populations Considered
There have also been some concerns voiced over how the vaccine may affect pregnant and breastfeeding women, who were not included in BioNTech/Pfizer or Moderna’s trials. It is important to note though that vaccines have been given to this demographic for decades without any safety concerns. Despite this, the FDA’s EUA for BioNTech/Pfizer’s vaccine does not make any particular mention of this group, but does say that Pfizer should gather long-term data on the administration of the vaccine in pregnant women.
Expectant mothers are also being given the option of getting the vaccine and should discuss it with their doctor. The vaccines may eventually be evaluated in pregnant women as well as children.
Animal Studies
Pre-clinical animals studies were largely conducted alongside clinical testing. While animal studies are useful, they have inherent issues, the main one being that animals such as mice — which are typically used in studies — are physiologically different from humans.
This poses to be a general issue in the bridge between pre-clinical and clinical trials. Not only do about 80 to 90 percent of drugs and vaccines that pass animal studies go on to fail in human clinical trials, but almost 90 percent fail in animal experiments and do not even progress to human testing. The latter represents a potentially significant gap where some drugs may end up, as they may very well be effective in humans but don’t get tested in people because they did not work in animals. Also, drugs and vaccines are lumped together in these numbers, so the success rates could be different for vaccines.
However, Moderna conducted pre-clinical studies in mice and monkeys, which showed that immune responses were elicited by the vaccines in the animals and provided protection against infection in the lungs and noses. Moderna is now looking at the effects of its vaccine on fertility and pregnancy in the animals.
mRNA Vaccines are New — Are They Safe?
BioNTech/Pfizer and Moderna’s vaccines are both based on mRNA (messenger RNA) technology. Although these will be the first mRNA vaccines approved for any disease to date, mRNA vaccine and drug technology has been around for well over a decade.
What is mRNA?
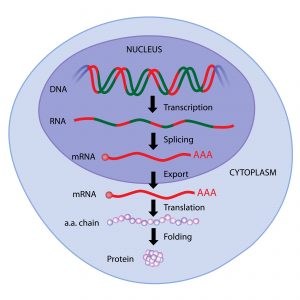
The central dogma in molecular biology is that the flow of genetic information goes from DNA to RNA to protein.
mRNA carries the code for making a specific protein based on the DNA blueprint.
In the nucleus of a cell, mRNA is transcribed from DNA, and is essentially a reverse photocopy of it. The sequence of mRNA is complementary to the portions of DNA (i.e. genes) it is transcribed from.
After mRNA is transcribed from DNA in the nucleus, it travels outside into the cytoplasm where it feeds its encoded instructions to proteins called ribosomes, which manufacture the given protein.
Although mRNA is a nucleic acid similar to DNA, it has one strand instead of two, making it much less stable than DNA. This reflects its intermediary, temporary status as a messenger molecule.
Bioengineered mRNA molecules are designed to code for a specific protein of interest and injected into the body as a drug or vaccine. mRNA technologies optimize the stability of mRNAs including developing effective packaging and delivery vehicles for it such as lipid-based nanoparticles.
In the case of a COVID-19 vaccine, the vaccine contains mRNA that codes for a portion of the spike protein.
Once in the body, the mRNA will lead to the production of the viral spike protein. The protein will be seen as foreign and activate the immune system, which will produce specific neutralizing antibodies against it, as well as memory immune cells that will ‘remember’ the spike protein and be ready to launch an attack against it if it sees it again in the future.
This mRNA vaccine principle is the same as any other vaccine including live attenuated vaccines which involve injection of a weakened form of a live virus, which some see as risky. On the other hand, mRNA vaccines don’t involve an active, full-form virus.
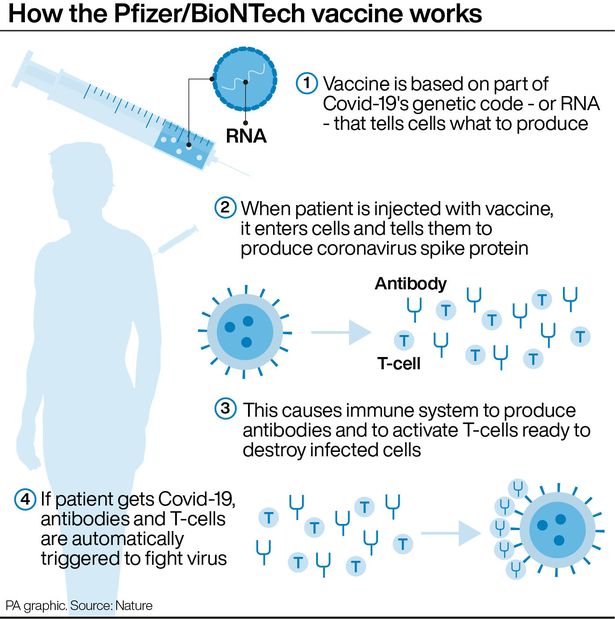
Can mRNA Alter DNA?
No, mRNA cannot alter DNA. Once a molecule of mRNA enters a cell as a drug or vaccine, it gives the instructions for making the specified protein and is then quickly destroyed after doing its job. This gives it zero opportunity to go into the nucleus of a cell and alter DNA.
Remember, the flow is from DNA to RNA, and not RNA to DNA. However, in some cases, viruses like retroviruses inject RNA into cells and is converted to DNA, which can be integrated into host DNA.
However, in a cell, mRNA is a specialized type of RNA that is produced and exported out of the nucleus after being transcribed from DNA, with no evidence of it being able to go back in. It has protective sequences of nucleic acids added to it for stability, which may also prevent its re-entry into the nucleus.
Side Effects
Aches and a slight fever? Congratulations, the vaccine is working.
No serious adverse reactions have been found in both BioNTech and Moderna’s COVID-19 vaccine trials.
The side effects of the vaccine – which include redness and temporary pain at injection site, slight muscle aches, headache and fever — are usual, mild and resolve quickly. These side effects are actually a good sign as they show that the vaccine is working. In any infection, aches, pains and fevers are an indication that your body is fighting a foreign invader.
A vaccine is the same idea as it introduces a small part of a virus into your body so that it can build immunity against it. So the aches and pains are normal and a good sign that your body is recognizing the vaccine as a foreign entity and launching an attack. Aches and a slight fever? Congratulations, the vaccine is working.
Any adverse side effects have been, and continue to be reported . This should lend confidence to the fact that the study processes are transparent and being conducted with due diligence.
Bell’s palsy
Alarm was raised over the occurrence of Bell’s palsy among four participants in BioNTech/Pfizer’s study that received the vaccine. In Moderna’s study, three people who got the vaccine developed the condition, which causes temporary facial paralysis thought to occur due to inflammation of the facial nerves. It can cause one side of the face or eyelid to droop. Only one person in the control placebo group in Moderna’s trial experienced the condition and none in Pfizer’s.
Most cases of Bell’s palsy are not serious and fully resolve on their own within a few days or weeks. Severe cases may require steroid or anti-viral treatments.
Health experts say that the occurrences were likely coincidental and not caused by the vaccine. It is thought that Bell’s palsy results from viral infection of the facial nerves due to reactivation of latent viruses such as chickenpox and some herpes viruses that remain in the body even after infections from them resolve. As such, while the exact causes of Bell’s palsy are not known, they may be related to infections, and thus the condition is found more commonly in people who have had a recent bout of the flu or a cold. It is also more common among people with diabetes and pregnant women.
In the general population, Bell’s palsy occurs in about 20 of every 100,000 people, which is a rate of 0.02 percent. Seven people experienced the condition out of the combined 37,000 that received the COVID-19 vaccines across both the BioNTech/Pfizer and Moderna trials — this translates to a rate of just under 0.02, comparable to the incidence in the general population. About 40,000 Americans get Bell’s palsy every year.
Hollywood actress Angelina Jolie revealed that she had Bell’s palsy a couple of years ago and made a full recovery.
Nevertheless, it is unusual that cases mostly occurred in the vaccine arm of the trial and as such, the FDA has said it will continue to closely monitor the trials for any more cases.
The bottom line is that adverse side effects have been, and continue to be reported if they arise. This should lend confidence to the fact that the study processes are transparent and being conducted with due diligence.
Long-Term Effects
While we don’t know the long-term effects of the vaccine, we also don’t know the long-term consequences of COVID-19. So far there are reports that it could cause lasting damage to the lungs, heart and brain as the virus can directly invade these tissues.
The most common symptoms after acute COVID-19 are fatigue, dyspnea, brain fog, joint pain and chest pain. COVID ‘long-haulers’ are people who cannot shake off a lot of these symptoms weeks and even months after infection. These people experience lingering symptoms as a result of the virus itself, or from treatment interventions such as intubation for ventilators.
There are also anecdotal reports of the female menstrual cycle being thrown off, which could have implications for fertility.
Ingredients & Allergies
In addition to mRNA as its active ingredient, BioNTech/Pfizer’s vaccine contains standard non-medicinal packaging ingredients. This includes four lipid nanoparticles that help the mRNA enter cells, a small amount of polyethylene glycol (PEG) attached to one of the lipids and salts.
Here is the full list of ingredients for the BioNTech/Pfizer vaccine:
mRNA (active ingredient)
Lipids
(4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate)
2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide
1,2-Distearoyl-sn-glycero-3-phosphocholine
Cholesterol
Salts
Potassium chloride
Monobasic potassium phosphate
Sodium chloride
Dibasic sodium phosphate dihydrate
Sucrose
Moderna’s vaccine has much of the same ingredients as BioNTech/Pfizer’s vaccine.
There were some concerns raised over PEG, which is a common compound found in detergents such as soaps and shampoos and is also used as a food additive, as well as a laxative at higher concentrations. A small fraction of people may be allergic to PEG.
In light of this, Pfizer has advised people with PEG allergies to not get the vaccine. This is a sensical approach compared to the nonsensical hearsay that 70 percent of people develop life-threatening immune reactions to PEG. These claims were found to be baseless.
If anything, people should be more worried about the PEG in their shampoos and cakes rather than the small amount they’ll get in a one-time shot or two of the vaccine, which will be quickly cleared from the body.
Vaccine Contains Fetal Cells
Many drugs and vaccines are developed using a kidney cell line called HEK-293T that was derived from an aborted fetus. It is one of the most widely used cell lines in research as the cells replicate rapidly and readily take up viral vectors and genomic material like DNA and RNA. Their use has led to many scientific discoveries about the function of thousands of genes and proteins.
So while BioNTech/Pfizer’s vaccine used this cell line to make the vaccine, the vaccine itself does not contain any remnants of the cell line. Cells are injected with the mRNA to amplify it i.e. make many copies. The mRNA is then purified using centrifugation and biochemical extraction methods. So there are no traces of cellular material in the vaccine.
General Vaccine Concerns
Vaccination has been around for well over 200 years, with the first vaccine having been introduced in 1796 by English physician Edward Jenner for smallpox. Since then, vaccines have helped eradicate other serious and deadly diseases such as polio, tetanus, rubella, measles, diphtheria, rotavirus and hepatitis A and B among several others. Vaccines have been tried, tested and proven.
In many school systems around the world, children are required to present a vaccination card, with a checklist of mandatory shots beginning in infanthood and reminders for top-up booster shots. A COVID-19 vaccine card would be no different.
Vaccines and Autism
The anti-vaxxer (i.e. anti-vaccine) movement gained popularity thanks (or rather, no thanks) to a handful of American celebrities with a poor/lack of understanding of science. The movement unfortunately neatly folded in with the newly formed anti-mask crusade that erupted in small, but loud pockets during the pandemic.
The movement was bred out of a belief that vaccines are linked to the increased incidence of autism. Autism spectrum disorder (ASD) has been on the rise since the 1990s, which is likely due to a combination of changes in diagnostic criteria as well as increased awareness, translating into more diagnoses of the condition.
And although a 1998 study outlined that a handful of children purportedly developed ASD after receiving the measles-mumps-rubella (MMR) vaccine, with a link to gastrointestinal issues, the study was found to be flawed as it did not have a control group among other things. As a result, the published paper was retracted. Numerous studies since then have investigated the link and failed to find an association between autism and vaccines, much less a causal effect.
Vaccines and Mercury
Questions were also raised over a vaccine ingredient called thimerosal, a mercury-based preservative used in multi-dose vials of vaccines. Research has shown that the compound does not cause ASD. Before the conclusive studies, thimerosal was actually eliminated or reduced in all childhood vaccines (except multi-dose flu shots, for which there are alternatives) between 1999 and 2001 as a precaution and as part of more widespread efforts to reduce any type of mercury exposure.
Vaccines are one of the most closely studied medical interventions in science and medicine. People are followed for years and decades, and retrospective studies can look back at people who have been vaccinated to study the effects of a vaccine. So there is a lot of data on vaccines. While the technologies and constituents may change i.e. mRNA vs. DNA vs. whole virus, the theory remains the same and vaccines will continue to be tested rigourously.
The World Watchdog
Despite all the myths and conspiracy theories doing the rounds, it would be hard to believe that any government or corporation would pull off a pandemic stunt of this magnitude.
Shutting down entire economies is hardly worth the price of selling a vaccine. And I’m sure there are more efficient ways of tracking persons of interest over following Fred’s Friday night at the bingo hall (post-pandemic of course) or tracking my Tim Horton’s coffee (or rather steeped tea) run.
Also the fact that the vaccines are being given to the most sensitive and susceptible populations first such as older adults in long-term care, should bring some sense of assuredness, and salutes for the elderly, as well as healthcare staff who have also been rolling up their sleeves for the vaccines.
In our modern-day technological world, complete with hacks and tracking capabilities, a pandemic and vaccine ‘scam’ would be hard to cover as the world is watching with its eyes peeled. With information and resources at our fingertips and unprecedented global interconnectedness through the internet and social media, the world is watching from anywhere and everywhere.
Science and Fact
It is important to be pro-science.
The information age has also bred an era of misinformation and rampantly running conspiracy theories. To help weed out the untruths, it’s important that evidence and facts not only come from ‘reputable’ sources but also multiple sources.
Consensus is formed out of rigour, reproducibility and the confidence that stems from them — these concepts are central to the scientific method. In a research lab, an experiment is only good if it can be repeated at least three times — the power of three, or more.
Consensus is not uniformity, it is unanimity based on systems of checks and balances. Peer-review is central to science today, as independent scientists and researchers around the world work to scrutinize data and reports coming out on the daily, ensuring that the science is robust.
Checks and balances are also based on principles of harm reduction in medicine, where the benefit of treatment is evaluated against the risk of any harm that it may cause — a simple cost-benefit analysis.
At the end of the day, it is important to be pro-science. And along with that, I am pro-treatments such as vaccines that have prevented me from getting potentially debilitating and deadly diseases in childhood and beyond.
So until someone can show me even a smidgeon of evidence about any vaccine having a microchip swimming in it, I’m sticking to the facts.
You May Also Like

We Must Cancel Christmas This Year if We Want to Have One Next Year
December 25, 2020
The COVID-19 Crisis in Long-Term Care and What to Do About It
February 16, 2021